YOU – Getting Involved in Research
The main objective of orthopaedic research is to improve lives...What does participating in research mean?
How you participate in research can vary and usually depends on the Study.
It may be as simple as giving consent for swabs to be taken during surgery and sent to a laboratory for testing. Or, you might come in for a quick examination, have a scan (e.g. MRI, CT or ultrasound) and/or complete some questionnaires on what you can and can’t do with your shoulder or elbow. This generally happens before and after surgery – so that we can compare.
How long will it take?
Most of our research requires 1 or 2 visits and/or 2 scans of about 20 to 30 minutes, so all up about an hour or two. Some studies follow people over a short period of time and others over a longer period ie there may be a break of 6 months to 1 year or more between visits, depending on what is of interest. This is to allow for normal recovery and return to pre-injury activities and helps to ensure the comparisons we make are reliable and valid.
Finding out about a study
Usually your treating surgeon will introduce a Study you may be suited for during your consultation. If you are interested, you will be contacted by an SSRI researcher (Jasmin or one of the surgeons undertaking fellowship at SSRI) and the Study will be explained to you in greater detail. You will also be given a copy of the Patient Information Sheet and Consent Form (or “PISCF”). You will then be given sufficient time to read through the PISCF, consult with your family, friends or your GP and had any questions answered by SSRI to your satisfaction. Only then should you wish to go ahead, will you be asked to consent to participate in the Study.
Patient Information Sheet and Consent Form
As mentioned, the Patient Information Sheet and Consent Form (or “PISCF”) is often the main source of information regarding a Study available to participants and potential participants. Typically, the PISCF introduces the Study, gives some background to the relevant disease or disorder and rationale for the study, details the Study’s designincluding which groups will be looked at, what measures or assessments will be done, exactly what is expected of participants, what the risks and benefits are (if any) all in plain English or “layman’s terms”. Our Patient Information Sheet and Consent Forms and any other documents we use for research are prepared in strict accordance with guidelines set by The National Health and Medical Research Council. The Council’s guidelines are continuously fine-tuned and improved with the aim of ensuring participants are able to make fully informed, voluntary decisions to consent and participate in research.
Giving your consent
Regardless of the type of study and type of participation it may require, you will be asked to give your informed consent before anything happens. “Informed” consent means you are given as much information as possible (and are therefore ‘informed’) and therefore best able to decide whether or not to participate. This information is generally provided in written form i.e. the PISCF mentioned above. The PISCF relates specifically to the Study and details what your participation involves, how any information you provide is used and protected as well as information regarding benefits, risks, costs and compensation. We cannot and will not use any of your information for any study without your consent and it is also important to know that consent can be given then revoked at a later date. This means you are free to change your mind (for whatever reason) regarding participating in a study and the decision to do so will bear no consequence on the treatment you receive either now or in the future from your treating surgeon or anyone else at SSRI. To change your mind regarding (“revoke”) consent you have given for a study you simply need to contact a member of the study team in writing (eg email). To make this simple, a“Revocation of Consent” form with instructions is attached to every PISCF which will be provided to you to keep for your records.
Privacy and Confidentiality
Researchers take privacy and confidentiality very seriously. SSRI is no different. We do everything possible to protect the privacy and confidentiality of people who participate in our research. Any and all data we collect is managed sensitively, respectfully and securely. Password encrypted programs and computers, locked cabinets and de-identification of data wherever possible are just some of the means by which we ensure privacy and confidentiality for participants. Information is used and known to only those who need to know and only passed on to parties outside of SSRI with the express permission of the participant. After a study has concluded and all necessary follow-up complete, data is stripped of any identity, archived and then securely shredded or otherwise disposed of after the requisite storage period (generally 5 to 10 years dependent on the type of study). At any time during a study should you decide you no longer wish to participate or no longer wish your data to be kept by SSRI you can request in writing for it to be removed and this will be respected. SSRI has developed its own Privacy Policy which can be viewed here.
BENEFITS
Usually there are no direct benefits of participating in research beyond the satisfaction one feels for having been of assistance. In most cases research participants are happy with this and do not expect anything more. In some instances, a participant may benefit later in life if they are once again a patient and require surgery and/or rehabilitation and the techniques for same have come about from or been improved by research. In sum, the benefits derived from current patients participating in research are understood to be enjoyed by future patients and the broader community.
RISKS
Risk is taken very seriously by researchers and ethics committees alike. Any and all foreseeable risks arising from a study and what researchers will do should things go wrong are fully explained in the Patient Information Sheet and Consent Form for each study. It is entirely up to you whether or not you choose to be involved with a study after receiving relevant information regarding risk and having opportunity to have any and all questions answered to your satisfaction. As mentioned previously, this is the giving of informed consent. Generally speaking, research projects undertaken at SSRI are of “low or negligible risk” to the participants. This means that there is no foreseeable risk of harm of discomfort for you if you take part in the research.
COSTS AND COMPENSATION
Research participation is broadly understood and very much appreciated as an act of altruism and as such most people do not expect to be paid for their participation. Of course conversely, assisting with research should also not cost a participant anything. What this means is, should we, for example, request you undergo an MRI scan specifically for research, we would pay your bill at the radiology practice for that scan. In some cases, travel costs incurred as part of research may also be covered by Sydney Shoulder Research Institute. Specific cost and compensation information is available within the Patient Information Sheet and Consent Form for each study. It is important to note, however, that you will still be responsible for the routine costs of your treatment and any surgery you require, regardless of whether you participate in research or not. This would be the case of any patient and is of course fair and reasonable.
PARTICIPATING IN RESEARCH
SAMPLE QUESTIONNAIRE
An example of a questionnaire commonly used in shoulder and elbow research is the American Shoulder and Elbow Society rating scale (“ASES”).The scale covers pain, stability and function in either a simple “yes / no” or “0” to “3” rating scale.
Quick and easy to administer this questionnaire and others used at SSRI have robust validity and reliability across various populations of patients with shoulder complaints.
Other orthopaedic outcomes used at SSRI are under licence through Socrates Orthopaedic Outcomes Software or through our own individual licences with the test publishers.
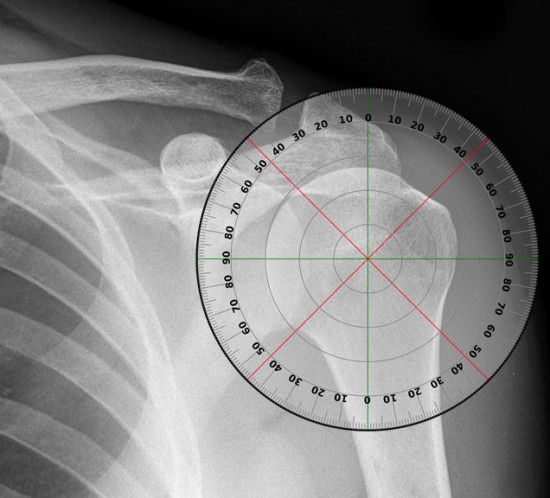
SAMPLE EXAMINATION
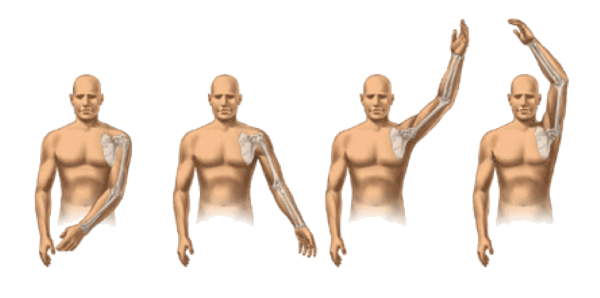
SHOULDER JOINT RANGE OF MOTION
A sample examination performed during a study is the simple checking of range of motion of your shoulder.
Range of motion is divided into 4 different areas: abduction, forward elevation, internal rotation and external rotation.
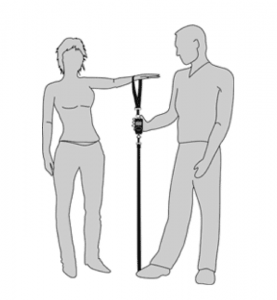
SHOULDER STRENGTH
Loss of strength in the shoulder is very common with shoulder injuries and disease such as osteoarthritis. To test shoulder strength we use a device known as a dynamometer. The person being tested has their wrist in the strap and pulls their arm up against resistance from the examiner who has their foot on the strap and the device in between measures the strength in kilograms.