Background
The Nexel is a recent implant and there are few studies that show how it behaves in the long term with osteolysis
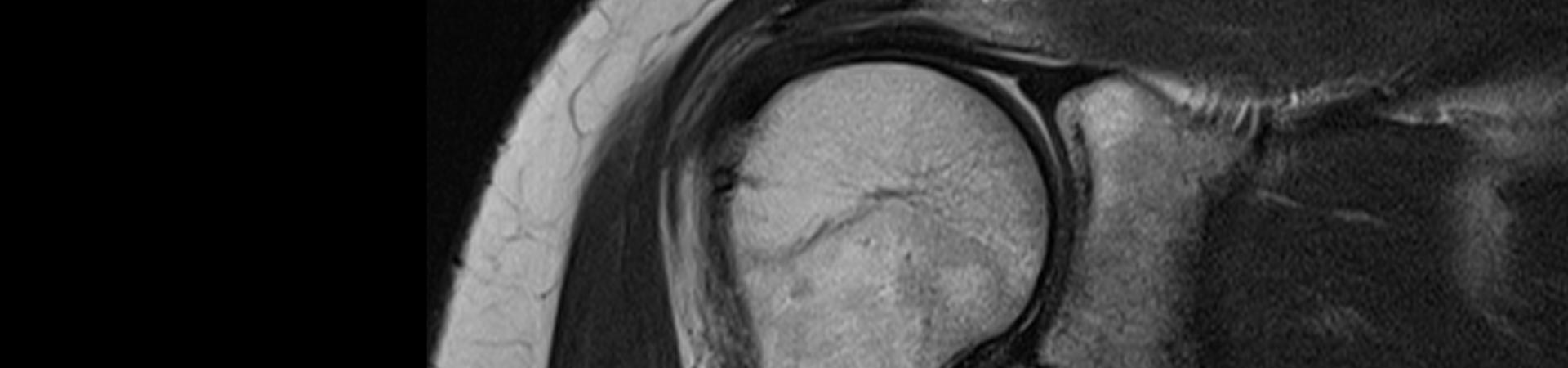
Total Elbow Arthroplasty (TEA). Some of the known modes of failure of TEA are septic loosening, polyethylene wear, osteolysis around the implant and peri prosthetic fracture.
Possible mechanisms for osteolysis are believe to be polyethylene wear secondary to poor implant positioning and edge loading of the polyethylene, flexion impingement, creation of debris and secondary foreign body reaction and component polymethylmethacrylate surface finish (Jeon, Morrey, & Sanchez-Sotelo, 2012).
Loosening of the ulna component is a major cause of revision in Total Elbow Arthroplasty (TEA). The Nexel total elbow arthroplasty was developed with a new bearing design that allows greater contact area with better load sharing and lower edge loading compared to the Coonrad/Morrey arthroplasty (King, et al., 2019).
There is although a potential for impingement in the extreme of range of motion of the elbow in extension and flexion which can produce macroparticles and secondary, increased rate of osteolysis. We hypothesize that the osteolysis rate around the prosthesis will be overall the same when care is taken during the procedure to avoid impingement.
The Nexel prosthesis is a recent implant and there are no available studies to show how it is behaving in the long term with osteolysis. Elbow arthroplasty is not a frequent procedure. We want to use this as an opportunity to review our results and to share our experience with this implant.